Abstract
Congenital PHF6 mutations (PHF6MT) cause the X-linked neurodevelopmental disorder, Börjeson-Forssman-Lehmann syndrome (BFLS). In contrast, acquired PHF6MT are frequently detected in hematologic neoplasms including acute myeloid leukemia (AML), myelodysplastic syndrome (MDS), and T-cell acute lymphoblastic leukemia (T-ALL). Indeed, PHF6 is highly expressed in brain/developing CNS as well as in hematopoietic subpopulations suggesting its role in neurogenesis and hematopoiesis consistent with the neurologic BFSL presentation of the germline and leukemias with somatic mutations of this gene. This study focuses on involvement of PHF6 lesions in AML. Here, we hypothesize that there are functional links between lymphoid and myeloid neoplasia (MN) with PHF6-deficiency that can be instructive in terms of the impact of this gene as a tumor suppressor gene (TSG).
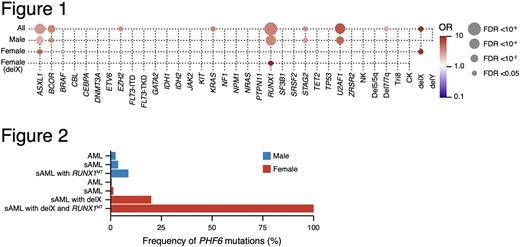
In total, we analyzed sequencing results of 6886 AML cases, including 616 cases from open sources such as The Beat AML Master dataset (Tyner JW , Tognon CE, Bottomly D, et al. Functional genomic landscape of acute myeloid leukaemia. Nature. 2018;562(7728):526-531). We first investigated the clinical phenotype associated with PHF6MT. We detected a total of 111 (1.6% of cases) PHF6MT. While frameshifts were found across all the coding regions, missense and stop-gains were concentrated in the two PHD-type zinc finger domains and were overlapping between other MN and the previously reported T-ALL cases. PHF6MT were significantly more common in male cases (2.3 vs. 0.8%; p<.001) similar to other X-genes such as UBA1, ZRSR2, and STAG2 but different from ATRX, PIGA, and KDM6A (UTX). When comparing primary AML (pAML) with secondary AML (sAML), sAML had more PHF6MT than pAML (2.9 vs. 1.4%; p<.001). While PHF6MT were not associated with chromosomal abnormalities in male cases, female cases with X chromosome deletion (delX) showed higher frequency of PHF6MT compared to females with diploid X chromosome (6.5 vs. 0.8%; p=.0019). Consequently, when delX were included, a total of 2.3% of female patients had PHF6 hits with 10% of them in a homo/hemizygous configuration.
While RUNX1, U2AF1, and ASXL1 mutations coincided with PHF6 hits in males, in female cases, delX and ASXL1 mutations were the most common associated defects (Fig.1). Interestingly, RUNX1 mutations (RUNX1MT) in females were enriched in cases with delX. As a result, PHF6MT were enriched into two distinct subclasses: while PHF6MT were enriched in male cases with sAML and RUNX1MT (8.8%), all female cases with sAML, RUNX1MT, and delX also acquired PHF6MT (Fig.2).
A higher frequency of PHF6MT in advanced MN than early diseases (e.g., low-risk MDS), suggestive of a secondary rather than a founder role of PHF6MT prompted us to study the clonal architecture of PHF6MT cases using variant allelic frequencies. RUNX1MT were dominant/co-dominant to PHF6 hits in the clonal evolution of AML in contrast to T-ALL, in which PHF6MT appears to be an earlier event. Similar relationship was found with regard to PHF6 hits and other myeloid gene mutations (e.g., ASXL1, DNMT3A, or SRSF2) suggesting a lineage-restricted TSG function of PHF6 in AML following myeloid commitment (consistent with the physiologic expression of PHF6 in pre-B, pre-T, and erythroid progenitors during hematopoietic ontogeny).
PHF6 mutations also affected AML patients’ outcome. Overall, PHF6MT showed worse outcome in male cases, but not in female cases. However, if PHF6MT cases also had RUNX1 mutations, the outcome was extremely low in both sexes. Unsupervised RNAseq analysis of PHF6 mutational status vs. expression pattern revealed that PHF6MT cases showed higher mRNA levels of several lymphoid genes (GCSAM, LY9, or BLNK). This finding was also confirmed by RNAseq of in-house PHF6 knock-out K562 and U-937 clones. Immunohistochemistry unveiled that LY9 was positive in illustrative PHF6MT cases and flow cytometry revealed the presence of lymphoid markers in 2/3 PHF6MT cases.
Our study confirms that PHF6MT are more common in male AML. Also we demonstrate the intricate relationships between PHF6MT and RUNX1MT in male sAML patients which extend also to female cases with delX. In AML, PHF6MT are a late event compared to T-ALL, in which they occur early in leukemogenesis. This is also in line with higher frequency of PHF6MT in sAML. Some PHF6MT cases have higher expression of lymphoid genes, which may be candidate for molecular targeted therapy.
Disclosures
Haferlach:Munich Leukemia Laboratory: Current Employment, Other: Part ownership. Maciejewski:Alexion: Consultancy; Apellis Pharmaceuticals: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal